In quantitative chemical analysis, precision is the bedrock of reliable results. Few pieces of laboratory equipment embody this principle more than the buret, a tool designed for the meticulous liquid measurement and delivery of a chemical solution. Mastering the scale on a burette is a fundamental skill for anyone involved in acid-base titrations or other volumetric analyses.
This guide provides a comprehensive walkthrough of the entire process, from initial setup to final calculation, ensuring every user measurement is as accurate as possible.
Understanding the Buret
A buret is a long, graduated glass tube with a stopcock at the bottom that allows for fine control over the flow of a solution. Unlike a measuring cylinder or graduated cylinders, which are designed for approximate measurements, a buret is calibrated to deliver a precise, variable volume.
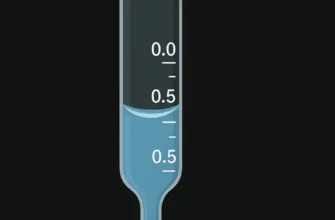
A key feature is its inverted measurement scale. The zero mark (zero scale) is at the top, and the numbers increase downwards. This design is intuitive for data collection during titrations because the reading directly shows the total volume delivered, a concept noted by educational resources from Viterbo University and the American Association of Chemistry Teachers.
While traditional glass burets are common, digital burettes are an increasingly popular alternative. These devices, often in the form of a Bottle Top Dispenser, eliminate manual reading errors but are more expensive.
Step-by-Step Guide to Buret Measurement
Achieving accurate results requires a methodical approach at every stage.
1. Preparation and Setup
Proper preparation prevents contamination and ensures the equipment functions correctly.
-
Cleaning: Wash the buret with laboratory detergent and a brush, then rinse it multiple times with tap water, followed by a final rinse with distilled water or deionized water.
-
Rinsing with Titrant: This is a critical step. Rinse the buret two to three times with a small amount of the titrant (e.g., sodium hydroxide) you will be using. This removes any residual laboratory water, preventing dilution of your standard solution.
-
Setup: Secure the buret vertically in a burette clamp on a ring stand. Place a waste beaker below the tip.
2. Taking the Initial Reading
The starting point for your measurement must be precise.
-
Filling: Using a funnel, perform an initial fill of the buret with your titrant to a level above the zero mark. Remove the funnel immediately to prevent drips from altering the volume.
-
Removing Air Bubbles: Open the stopcock fully for a moment to flush any air bubbles from the tip. A bubble-free tip is essential for an accurate delivered volume.
-
Recording the Initial Volume: Lower the liquid level until the bottom of the meniscus is on or just below the zero scale. It is not necessary to hit 0.00 exactly. The most important thing is to record the precise initial volume to two decimal places (e.g., 1.24 mL). Avoid parallax error by ensuring your eye is level with the meniscus.
3. Dispensing and Titration
This step requires control and careful observation.
-
Dispensing: Add the titrant to the receiving container (e.g., an Erlenmeyer flask). In the beginning, a steady stream is acceptable, but as you near the endpoint, slow the rate to a drop-by-drop addition.
-
Endpoint Detection: Watch for the indicator’s color change. The endpoint is reached at the first sign of a permanent change.
-
Waiting Period: After closing the stopcock, wait 15-30 seconds to allow the liquid clinging to the buret walls to drain down before taking the final reading.
4. Final Reading and Calculation
The last step is to record your final measurement and calculate the result.
-
Record the Final Volume: Read the final position of the meniscus to two decimal places, again ensuring your eye is level to prevent parallax error.
-
Calculate the Volume Delivered: The total volume of sodium hydroxide (or other titrant) used is the difference between the initial and final burette reading.
Volume Delivered = Final Reading – Initial Reading
For example, if your initial volume was 1.24 mL and your final reading was 25.86 mL, the delivered volume is 24.62 mL. This method of subtracting the initial reading from the final is why the scale on a burette seems to be a measurement backwards compared to a measuring cylinder.