The Acid Burette: A Practical Guide to Its Function and Use
Laboratory precision begins with selecting the right instrument for each specific task. In analytical chemistry, few pieces of equipment are as fundamental as the burette, and when working with corrosive liquids, the acid burette becomes an essential tool. This specialized instrument stands apart from its base counterpart through one critical design feature: its chemically resistant stopcock mechanism, engineered specifically to withstand the aggressive nature of acids and oxidizing agents while maintaining the precision required for accurate volumetric analysis.
Core Function: What an Acid Burette Is Designed to Do
Primary Role in Volumetric Analysis
The acid burette serves a fundamental purpose in analytical chemistry: to dispense a precisely known volume of acidic titrant with exceptional accuracy. This graduated glass tube, typically ranging from 10 to 100 mL capacity, enables chemists to control the addition of corrosive solutions drop by drop, achieving measurements accurate to 0.01 mL.
The Critical PTFE Stopcock Component
The defining characteristic of an acid burette lies in its PTFE (polytetrafluoroethylene) stopcock mechanism. This stopcock material offers exceptional chemical resistance, remaining inert when exposed to strong acids, bases, and solvents. Unlike traditional glass stopcocks that require lubrication and can seize when exposed to certain chemicals, PTFE stopcocks provide smooth, maintenance-free operation.
Design-Function Integration
The relationship between design and function becomes clear when considering the corrosive nature of many analytical reagents. A reactive valve system represents a significant liability when handling aggressive chemicals that could cause deterioration, contamination, or complete failure of the stopcock mechanism. The PTFE material’s resistance to chemical attack ensures consistent performance even with concentrated acids and oxidizing agents.
Key Differences: Acid Burette vs. Base Burette
The distinction between acid and base burettes often causes confusion in laboratory settings, but understanding their differences is straightforward when focusing on the stopcock material as the defining characteristic.
| Feature | Acid Burette | Base Burette |
|---|---|---|
| Stopcock Material | PTFE (Teflon) or glass with PTFE key | Traditional rubber-tube pinch valve with glass bead |
| Chemical Compatibility | Acids, oxidizing agents, organic solvents | Alkaline solutions, non-oxidizing solutions |
| Maintenance Requirements | Minimal – no lubrication needed | Regular inspection of rubber components |
| Corrosion Resistance | Excellent against acids and solvents | Rubber degrades with prolonged alkali exposure |
The rubber components in traditional base burettes can deteriorate when exposed to strong alkaline solutions over extended periods. In contrast, PTFE material maintains its properties even after prolonged exposure to aggressive chemicals, including 3% HCl, 3% NaOH, and 3% NaClO solutions for weeks.
Essential Laboratory Uses and Applications
Acid-Base Titration Procedures
The most common application involves determining the concentration of unknown bases by titrating with standardized acid solutions. The acid burette allows precise control of acid addition, enabling identification of equivalence points with high accuracy.
Redox Titration Applications
In redox titrations, acid burettes are essential when the titrant is an oxidizing agent such as potassium permanganate (KMnO₄). These titrations require the chemical resistance that only PTFE stopcocks can provide, as permanganate solutions can attack rubber components.
Standard Solution Preparation
Acid burettes play a crucial role in preparing standard acidic solutions where precise volume control is paramount. The ability to dispense exact volumes enables the creation of solutions with known concentrations essential for analytical work.
A Step-by-Step Guide to Using an Acid Burette Correctly
Preparation: Conditioning the Burette
Cleaning Protocol: Begin with thorough cleaning using laboratory detergent and a burette brush, followed by multiple rinses with tap water and a final rinse with distilled water. This removes any residual materials that could interfere with measurements.
Solution Conditioning: Rinse the burette 2-3 times with small volumes (5-10 mL) of the solution to be used. This critical step prevents dilution of the titrant by residual water, ensuring accurate concentration.
Filling: Avoiding Air Bubbles
Proper Filling Technique: Using a funnel, fill the burette to above the zero mark with your titrant. Remove the funnel immediately after filling to prevent additional liquid from dripping in.
Air Bubble Elimination: The most critical aspect involves removing all air bubbles, particularly from the tip. Open the stopcock fully to flush bubbles out under pressure from the filled burette. Persistent bubbles may require gentle tapping or momentary suction techniques.
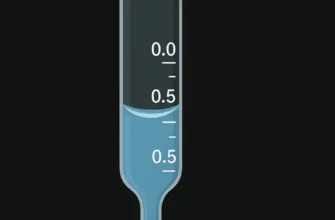
Reading the Meniscus: Achieving Precision
Eye Level Positioning: Position your eye level with the bottom of the meniscus to avoid parallax error. The graduation line closest to the meniscus should appear as a single solid line, not an oval.
Using Reading Cards: Employ a reading card (black line on white background) positioned approximately one marking below the meniscus to sharpen the bottom curve and improve reading consistency.
Two Decimal Place Accuracy: Read measurements to 0.01 mL by estimating the meniscus position between graduation marks. Remember that each graduation line has thickness (approximately 0.02 mL), and readings should reference the top of each marking.
The Titration Process: Control and Endpoint Detection
Flow Rate Control: Begin with steady flow for rapid approach to the endpoint, then reduce to drop-by-drop addition as the color change approaches. This prevents overshooting the equivalence point.
Endpoint Identification: Monitor for permanent color changes that indicate complete reaction. In permanganate titrations, the endpoint appears as a persistent light pink color.
Care and Maintenance for Longevity
Cleaning: Preventing Crystallization and Seizure
Immediate Rinse Protocol: Clean the burette with fresh water immediately after each use to prevent chemical deposits. Never allow solutions to dry inside the glassware, as dried chemicals can cause pitting or make removal extremely difficult.
Intensive Cleaning Procedures: For stubborn deposits, use appropriate cleaning agents designed for laboratory glassware. Disassemble the stopcock if necessary, cleaning all components thoroughly with deionized water.
Stopcock Maintenance: Unlike glass stopcocks that require regular lubrication, PTFE stopcocks need minimal maintenance. However, inspect the stopcock mechanism regularly for proper sealing and smooth operation.
Storage: Preserving Functionality
Proper Storage Position: Store burettes in an upright position in designated burette cabinets. Ensure the stopcock is positioned securely in the storage holes to prevent falling and breakage.
Stopcock Position: Store with the stopcock slightly open to prevent sticking, particularly important for glass stopcocks. PTFE stopcocks are less prone to this issue but benefit from this practice.
Environmental Conditions: Maintain storage in clean, dry conditions with temperatures between -20°C to +50°C and relative humidity between 5% and 95%.
Frequently Asked Questions
What is an acid burette?
An acid burette is a graduated glass tube equipped with a PTFE stopcock designed specifically for dispensing acidic solutions with high precision in analytical chemistry applications.
What is the difference between an acid burette and a base burette?
The primary difference lies in the stopcock material: acid burettes use PTFE stopcocks for chemical resistance, while traditional base burettes may use rubber-tube pinch valves.
Is burette used to measure acid?
Yes, burettes are used to measure and dispense acids accurately, particularly in titration procedures where precise volume control is essential.
Is acid in burette or pipette?
In acid-base titrations, the acid (titrant) is typically placed in the burette for controlled addition to the base (analyte) in the receiving flask.
Conclusion
The acid burette represents a fundamental tool in analytical chemistry, where precision and chemical compatibility converge to enable accurate quantitative analysis. The key value of an acid burette lies in its chemically resistant PTFE stopcock, which makes it the safe and reliable choice for handling corrosive liquids that would damage traditional rubber components.
Choosing the appropriate burette type is not merely a matter of preference but a critical aspect of proper laboratory technique that directly impacts data quality and safety. The acid burette’s specialized design ensures that corrosive titrants can be dispensed with the precision required for reliable, reproducible results in quantitative analysis, making it an indispensable instrument in any well-equipped analytical laboratory.