Navigating Ovulation Induction for Fertility
Navigating the path to parenthood can present unexpected challenges, with infertility affecting millions of couples worldwide. For many, the root cause lies in issues with ovulation, the monthly release of an egg from the ovaries. When this process is irregular or absent, conception becomes difficult. Fortunately, modern medicine offers a powerful solution: ovulation induction, a fertility treatment that uses specific medications to stimulate the ovaries and encourage them to develop and release one or more mature eggs, significantly increasing the chances of pregnancy.
What is Ovulation Induction?
Ovulation induction is a fertility treatment that uses hormonal medications to stimulate ovulation in individuals with ovulatory dysfunction. The primary goal is to correct anovulation (the complete absence of ovulation) or oligo-ovulation (infrequent or irregular ovulation). By promoting the growth of one or more mature follicles—the small fluid-filled sacs in the ovaries that contain eggs—this treatment helps ensure an egg is available for fertilization. It forms the foundation for many fertility treatments, from timed intercourse cycles to more advanced procedures like Intrauterine Insemination (IUI) and In Vitro Fertilization (IVF).
Who Benefits from Ovulation Induction?
The primary candidates for ovulation induction are individuals with ovulatory dysfunction, including those with irregular or absent menstrual cycles. This condition is often linked to Polycystic Ovary Syndrome (PCOS), a leading endocrine cause of female infertility. Women with hypothalamic amenorrhea (absence of a period due to hormonal issues affecting the hypothalamus) or other hormonal imbalances—such as elevated prolactin levels, thyroid disorders, or pituitary insufficiency—also benefit significantly from ovulation induction.
Additionally, ovulation induction may be used in cases of unexplained infertility, where it can increase the number of eggs released per cycle through a process called superovulation, thereby improving the odds of conception. The medication choice depends on the specific cause of ovulatory dysfunction and individual patient factors.
Understanding Ovulatory Dysfunction, Polycystic Ovary Syndrome (PCOS), and Your Fertility
The Foundation: How Ovulation Works and Why It’s Crucial
A normal menstrual cycle is a finely tuned hormonal symphony involving the hypothalamus, pituitary gland, and ovaries. The cycle begins when the hypothalamus releases gonadotropin-releasing hormone (GnRH), which signals the anterior pituitary gland to release Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH). FSH signals the ovaries to start maturing several follicles, each containing an immature egg. As these follicles grow, they produce estrogen. Once estrogen levels peak, the positive feedback to the pituitary triggers a surge of LH, which occurs approximately 34 to 36 hours before ovulation. This LH surge stimulates the most mature follicle (typically measuring 18-24 mm in diameter) to rupture and release its egg—an event known as ovulation. The egg then travels into the fallopian tube, where it can be fertilized by sperm. This precise hormonal cascade is essential for reproduction; without it, pregnancy cannot occur.
What is Ovulatory Dysfunction? Common Causes of Infertility
Ovulatory dysfunction is a broad term for any disruption in the ovulation process and represents one of the most common reasons for female infertility, accounting for approximately 20-40% of infertility cases. It can manifest as anovulation (complete absence of ovulation) or oligo-ovulation (infrequent, irregular ovulation occurring fewer than 9 times per year).
Causes are varied and can include:
-
Hormonal imbalances originating from the pituitary gland or hypothalamus
-
Thyroid disorders (both hyperthyroidism and hypothyroidism)
-
Elevated prolactin levels (hyperprolactinemia)
-
Lifestyle factors such as significant stress, extreme weight loss or weight gain, or excessive exercise
-
Primary ovarian failure or diminished ovarian reserve
However, Polycystic Ovary Syndrome (PCOS) is the single most prevalent cause of anovulation and ovulatory dysfunction in reproductive-aged women.
PCOS: A Deep Dive into a Primary Cause of Ovulatory Dysfunction
Polycystic Ovary Syndrome (PCOS) is a complex endocrine metabolic disorder affecting 8-20% of women of reproductive age (depending on diagnostic criteria used). It is characterized by:
-
Hormonal imbalances, particularly elevated androgens (male hormones)
-
Insulin resistance, present in approximately 50-70% of women with PCOS
-
Development of numerous small follicles in the ovaries, often visible on ultrasound as polycystic ovarian morphology
In women with PCOS, the ovaries often develop many small follicles but fail to select and mature a single dominant follicle for ovulation. This is due to abnormalities in the hormonal feedback loop, including elevated LH-to-FSH ratios and impaired FSH secretion patterns. This hormonal disruption interferes with the delicate FSH and LH balance required for ovulation, making PCOS a primary driver of infertility due to ovulatory dysfunction. The condition is also associated with metabolic complications, including increased risk of type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Before Treatment Begins: The Essential Fertility Workup
Comprehensive Fertility Testing for Both Partners
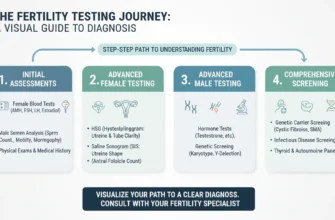
Before starting any ovulation induction medications, a thorough fertility evaluation is essential to ensure the treatment is appropriate, safe, and has the best chance of success. This comprehensive assessment is not just about the person who will carry the pregnancy—it is a team effort involving both partners.
For the female partner, testing typically includes:
-
Hormone level assessment: Blood tests on cycle days 2-4 to measure FSH, Luteinizing Hormone (LH), Estradiol, Progesterone, Prolactin, and Thyroid-Stimulating Hormone (TSH) to rule out hormonal imbalances
-
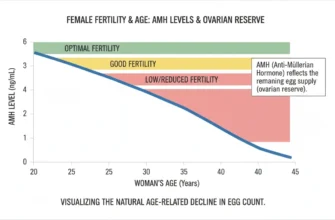
Anti-Mullerian Hormone (AMH) testing: Measures ovarian reserve (the number of remaining eggs); not cycle-dependent and can be drawn any day
-
Transvaginal ultrasound: Evaluates ovarian morphology, counts antral follicles (small follicles visible on ultrasound), and assesses uterine anatomy
-
Hysterosalpingogram (HSG) or Hysterosalpingo-Contrast Sonography (HyCoSy): Confirms that fallopian tubes are patent (open); using oil-based contrast may provide a therapeutic benefit with approximately 3.6 times greater odds of pregnancy compared to no HSG
-
Pelvic ultrasound: Evaluates the ovaries, uterus, and uterine cavity for abnormalities such as fibroids, polyps, or structural malformations
For the male partner, testing includes:
-
Semen analysis: A comprehensive evaluation assessing:
-
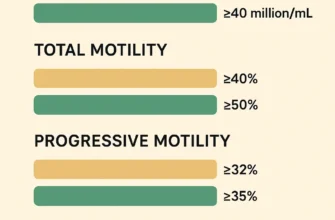
Sperm concentration: Measured in millions per milliliter; at least 15 million/mL considered acceptable
-
Total sperm count: At least 39-40 million total sperm in the ejaculate
-
Motility: Progressive motility (forward movement) of at least 40%
-
Morphology: At least 4% normally shaped sperm using strict criteria (WHO 2010 guidelines)
-
Presence of leukocytes, bacteria, or debris
-
Determining Suitability for Ovulation Induction: Criteria and Contraindications
The ideal candidate for ovulation induction has:
-
Confirmed ovulatory dysfunction (irregular or absent periods)
-
Preserved ovarian reserve (evidenced by normal or near-normal AMH and antral follicle count)
-
At least one patent fallopian tube allowing eggs and sperm to meet
-
A normal or near-normal uterine cavity without significant abnormalities
-
A male partner with adequate sperm parameters for natural conception or IUI (minimum 10 million total motile sperm for IUI)
Contraindications to ovulation induction include:
-
Ovarian failure or significantly diminished ovarian reserve (very high FSH, very low AMH, low antral follicle count)
-
Bilateral blocked fallopian tubes (preventing fertilization)
-
Significant uterine abnormalities such as severe scarring (Asherman’s syndrome) or major anatomical defects
-
Untreated male factor infertility with severe sperm parameters unsuitable for natural conception or IUI
-
Uncontrolled medical conditions that would make pregnancy high-risk
Setting Expectations: Realistic Outcomes and Personalized Treatment Plans
A critical part of the pre-treatment process is a detailed consultation with your fertility specialist to establish realistic expectations. Success rates vary significantly based on:
-
Patient age (younger patients have higher success rates)
-
The specific cause of infertility (PCOS has better prognosis than ovarian failure)
-
The chosen medication and protocol
-
Baseline ovarian reserve and other prognostic factors
Success rates range from approximately 30-50% per cycle with ovulation induction and IUI to higher rates with IVF. Your doctor will create a personalized treatment plan, outlining the specific drug, dosage, monitoring schedule, and expectations for each day of the cycle. Understanding that it may take 3-4 cycles to achieve pregnancy with ovulation induction and IUI (beyond which progression to IVF is typically recommended) is important for managing the emotional and financial journey of fertility treatment.
Letrozole (Femara): The First-Line Approach for Ovulation Induction
How Letrozole Works: Mechanism of Action as an Aromatase Inhibitor
Letrozole, marketed as Femara, is an oral medication classified as an aromatase inhibitor. While originally developed for breast cancer treatment in postmenopausal women, it has proven highly effective for ovulation induction. Letrozole works by temporarily blocking the enzyme aromatase, which converts androgens (testosterone and androstenedione) into estrogen in various tissues including the ovaries, fat, and adrenal glands.
This temporary reduction in estrogen production tricks the brain’s hypothalamic-pituitary axis into perceiving low estrogen levels, triggering increased production of Gonadotropin-Releasing Hormone (GnRH) from the hypothalamus and subsequently higher levels of FSH from the pituitary gland. This enhanced FSH signal provides stronger stimulation to the ovaries, encouraging the development of one or more mature follicles.
Key advantages of letrozole:
-
Short half-life (approximately 45 hours), meaning it is rapidly cleared from the body after treatment, limiting fetal exposure during critical organogenesis
-
Results in a thicker, more receptive endometrium compared to clomiphene citrate
-
Lower risk of multiple pregnancies (3-5% vs. 5-8% with clomiphene)
-
Better suited for individuals with insulin resistance or obesity
-
No adverse effects on endometrial receptivity seen with selective estrogen receptor modulators
The Letrozole Protocol: A Step-by-Step Cycle Guide
A typical letrozole cycle follows a clear, straightforward timeline:
Cycle Day 1: The first day of your period marks the beginning of the cycle. You contact your fertility clinic to schedule monitoring appointments and receive instructions.
Cycle Days 3-7 (Standard Protocol): You begin taking letrozole orally, typically at a dose of 2.5 mg, 5 mg, or 7.5 mg daily for 5 consecutive days. The dose is individualized based on age, BMI, ovarian reserve, and prior response to therapy.
Note: Some clinics use extended protocols (7-10 days) for resistant cases, and early-cycle initiation (days 2-6) has been investigated.
Cycle Days 10-12 (First Monitoring Ultrasound): You undergo a transvaginal ultrasound to measure follicle growth and assess the thickness and pattern of your uterine lining (endometrium). Optimal endometrial thickness ranges from 8-12 mm (acceptable range 7-14 mm) with a trilaminar (three-layered) appearance.
Continued Monitoring: Additional ultrasounds and blood tests measuring estradiol levels are performed as needed (typically every 1-3 days after initial scan) to track follicle development and assess ovarian response.
Ovulation Trigger Decision: Once ultrasound confirms at least one mature follicle measuring 18-20 mm or larger in diameter, ovulation is imminent. Your doctor may:
-
Allow ovulation to occur naturally, or
-
Prescribe an hCG trigger shot (such as Ovidrel) to time ovulation precisely
Conception Attempt: Following ovulation (which occurs naturally or approximately 36-40 hours after the hCG trigger shot), you will be advised on the best timing for:
-
Timed intercourse: Have intercourse on the day of the trigger shot and for 2-3 days thereafter to ensure sperm are present when the egg is released
-
Intrauterine Insemination (IUI): This procedure can be scheduled 24-42 hours after the trigger shot (optimal timing remains 34-42 hours in most protocols)
Progesterone Support: Your doctor may prescribe progesterone supplementation (via vaginal tablets, gel, or injection) beginning after ovulation to support the corpus luteum and prepare the endometrium for implantation.
Pregnancy Testing: A home urine pregnancy test is typically performed approximately 10-14 days after ovulation, with blood beta-hCG testing to confirm positive results.
Letrozole for PCOS: Specific Benefits and Evidence
For individuals with Polycystic Ovary Syndrome, letrozole is now widely considered the first-line treatment according to the 2023 International Evidence-based Guideline for PCOS, often outperforming the older medication, Clomiphene Citrate (Clomid). Landmark studies, including the large randomized Legro trial, have demonstrated that women with PCOS treated with letrozole have:
-
Higher ovulation rates (approximately 75-80% ovulation rate)
-
Higher live birth rates compared to clomiphene citrate
-
Significantly higher live birth rates in obese women (BMI ≥ 30 kg/m²), with rates approximately doubled compared to clomiphene
-
Lower rates of multiple gestations (3-5% vs. 5-8% with clomiphene)
-
Improved endometrial thickness and quality due to its mechanism of action
These benefits are attributed to letrozole’s short half-life, which results in rapid clearance from the body, allowing for a more physiologic FSH response and, importantly, a thicker and more receptive endometrial lining compared to clomiphene, which has anti-estrogenic effects on the endometrium.
A 2024-2025 meta-analysis of multiple randomized controlled trials confirmed no increase in congenital anomalies, miscarriage rates, or adverse perinatal outcomes with letrozole conception compared to natural conception or other fertility treatments.
Complementary Treatments for PCOS with Letrozole
For patients with PCOS, particularly those with documented insulin resistance, physicians often prescribe Metformin (typically 500-1000 mg two to three times daily, started at least 2-3 months before ovulation induction) as an adjunct to letrozole. Metformin is an insulin-sensitizing agent that:
-
Helps regulate blood glucose levels
-
May restore more regular menstrual cycles in some women
-
Reduces the risk of OHSS by approximately 63% according to Cochrane systematic reviews
-
Improves the ovulatory response and pregnancy rates in insulin-resistant women
Using metformin in conjunction with letrozole can improve both ovulation rates and clinical pregnancy rates compared to letrozole monotherapy in select patient populations. Research shows cumulative conception rates increase from approximately 41-47% with letrozole alone to 47-56% with letrozole plus metformin.
Gonadotropins: Injectable Hormones for More Intensive Ovulation Induction
What are Gonadotropins? Directly Administering Follicle-Stimulating Hormone (FSH)
Gonadotropins are powerful injectable medications that contain purified or recombinant forms of Follicle-Stimulating Hormone (FSH), sometimes combined with Luteinizing Hormone (LH). Unlike oral medications like letrozole or clomiphene, which indirectly signal the body to produce more FSH through hormonal feedback, gonadotropins directly deliver these hormones into the bloodstream via subcutaneous injection (beneath the skin).
This direct hormonal stimulation bypasses the brain’s feedback loop entirely and provides a much more potent signal for follicle development than can be achieved with oral medications. Gonadotropins come in several formulations:
-
FSH-only preparations (such as Gonal-F, Follistim, Bravelle): Appropriate for most ovulation induction cases, especially WHO Group II anovulation (like PCOS)
-
FSH + LH combinations (such as Menopur, Repronex): Used for women with WHO Group I anovulation (hypothalamic amenorrhea) who require endogenous LH stimulation
-
Recombinant vs. urinary-derived formulations: Both are effective; recombinant formulations (r-FSH) are now standard and offer greater purity and consistency
When Gonadotropins are Used: Indications for Injectable Medications
Gonadotropins are typically used in several clinical scenarios:
-
Failed response to oral medications: Patients who do not achieve ovulation with adequate doses of letrozole or clomiphene (approximately 15-25% of PCOS cases)
-
Clomiphene or letrozole resistance: Documented failure to develop a mature follicle despite appropriate dosing
-
Controlled ovarian hyperstimulation for IUI: Producing 2-4 mature follicles (compared to 1-2 with oral agents) to increase pregnancy chances in couples with:
-
Unexplained infertility
-
Mild male factor infertility
-
Mild endometriosis
-
-
In Vitro Fertilization (IVF) cycles: Higher doses of gonadotropins are used to retrieve multiple eggs for fertilization and embryo selection
-
Rapid cycle progression needed: When time to pregnancy is critical (advanced maternal age, low ovarian reserve)
The Gonadotropin Protocol: A Detailed Step-by-Step Approach
A gonadotropin cycle is more intensive and requires more frequent monitoring and patient contact than oral medication cycles:
Cycle Days 2-3: After a baseline transvaginal ultrasound confirms the absence of cysts and appropriate follicular development (typically performed on cycle day 2 or 3), you begin daily subcutaneous injections of the prescribed gonadotropin dose. Starting doses typically range from 75-150 IU daily using a step-up protocol (preferred for PCOS) rather than step-down.
Cycle Days 5-7 (Initial Monitoring): Starting around day 5-7 of injections, you begin frequent monitoring visits (typically every 1-3 days). Each monitoring appointment includes:
-
Transvaginal ultrasound: Counts the total number of developing follicles, measures their diameter (tracking the lead follicles), and assesses endometrial thickness
-
Serum hormone blood tests: Primarily measuring estradiol levels, which reflect the degree of follicle activity and response to medications
-
Assessment for signs of excessive response or other complications
Ongoing Dose Adjustments: Your fertility specialist carefully analyzes the data from each monitoring appointment and adjusts your medication dose based on:
-
Follicle growth rate and number of follicles
-
Estradiol levels and their rate of increase (excessive daily increases >100% are concerning for OHSS)
-
Endometrial thickness and appearance
-
Your individual response pattern and risk profile
If follicle growth is too slow (fewer than expected follicles or slow growth), the medication dose may be increased by 37.5-75 IU. If the response is too robust (too many follicles developing, excessive estradiol levels, or endometrial thickness concerns), the dose may be decreased or the cycle may be canceled to prevent severe OHSS or reduce multiple pregnancy risk.
Trigger Shot Administration: When follicles reach maturity (at least 1-2 follicles measuring 17-20 mm in diameter, with optimal endometrial thickness ≥8 mm), you will be instructed to administer an hCG trigger injection (typically 5,000-10,000 IU) to finalize egg maturation and reliably trigger ovulation.
Timed Intercourse or IUI: Timed intercourse or IUI is scheduled approximately 34-42 hours after the hCG trigger shot (traditional timing 36 hours; some data suggests 42 hours may optimize outcomes). This timing aligns with the expected ovulation window of 36-40 hours post-trigger.
Side Effects and Risks Associated with Gonadotropins
Because gonadotropins are potent and directly stimulate the ovaries, they carry a higher risk profile than oral medications. The most significant concerns include:
1. Ovarian Hyperstimulation Syndrome (OHSS)
OHSS is an exaggerated, potentially serious response to ovarian stimulation medications characterized by:
-
Mild-moderate OHSS: Abdominal bloating and mild-to-moderate discomfort, mild nausea, mild weight gain
-
Severe OHSS: Significant abdominal pain and distension, persistent nausea and vomiting, rapid weight gain (>2-5 lbs per day), decreased urination, shortness of breath, or dizziness
While most cases resolve spontaneously, severe OHSS can require hospitalization and intensive management. Primary risk factors include:
-
Young age
-
Low body mass index (BMI <25)
-
PCOS diagnosis (highest risk)
-
High antral follicle count (≥24 follicles)
-
High AMH levels (>3.36 ng/mL)
-
Prior OHSS history
Prevention strategies include using the lowest effective gonadotropin dose, employing step-up dosing protocols, regular monitoring with dose adjustments, using metformin (which reduces OHSS risk by ~63%), GnRH agonist triggering (Lupron) instead of hCG in high-risk cases, or “coasting” (temporarily stopping injections while continuing monitoring).
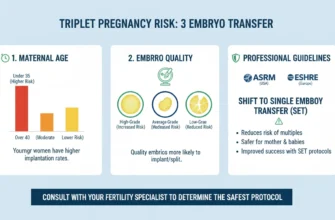
2. Multiple Gestations (Twins, Triplets, or Higher-Order Multiples)
The risk of multiple pregnancies is substantially higher with gonadotropins than with oral medications, particularly when multiple mature follicles are present:
-
Letrozole: 3-5% multiple pregnancy rate
-
Clomiphene citrate (single agent, per guidelines): 3.8% overall (2.4% when BMI <35)
-
Gonadotropins: Up to 32% multiple pregnancy rate, with higher risks when more follicles are triggered
Multiple pregnancies carry increased risks for both mother and fetuses, including gestational diabetes, preeclampsia, premature delivery, and fetal complications. Careful monitoring and individualized cycle management can help minimize this risk through appropriate dose titration and cycle cancellation if excessive follicle development occurs.
3. Other Potential Adverse Effects
-
Injection site reactions: Mild bruising, pain, or redness
-
Headache and mood changes
-
Mild gastrointestinal symptoms
-
Allergic reactions (rare with recombinant preparations)
The Critical Role of Monitoring During Ovulation Induction
Why Monitoring is Essential: Optimizing Outcome and Mitigating Risk
Monitoring is a non-negotiable component of any ovulation induction cycle, especially with injectable gonadotropins. Its dual purpose is to:
-
Maximize the chances of success: By precisely tracking follicle development, physicians can optimally time the ovulation trigger and subsequent intercourse or IUI procedures, ensuring sperm are present when the egg is released
-
Minimize risk: By identifying an excessive ovarian response early, the clinical team can adjust medication doses, implement preventive strategies, or cancel the cycle to prevent severe OHSS or manage multiple pregnancy risk
Without careful monitoring, ovulation induction carries substantially higher risks of both treatment failure and serious complications.
Monitoring Techniques Explained
The two primary tools for monitoring ovulation induction are:
1. Transvaginal Ultrasound
This specialized imaging technique provides a clear, detailed view of the ovaries and uterus by inserting a small ultrasound probe into the vagina. During monitoring, the ultrasound is used to:
-
Count the number of developing follicles in each ovary
-
Measure the diameter of each follicle (measured in millimeters)
-
Track the dominant follicles (typically the one or two largest) that will likely ovulate
-
Assess the thickness, pattern, and vascularity of the endometrium to determine receptivity
-
Evaluate the uterine cavity and fallopian tubes to rule out abnormalities or structural issues
Transvaginal ultrasound is preferred over abdominal ultrasound for ovulation monitoring because it provides superior image quality and clarity of ovarian structures.
2. Bloodwork: Hormone Level Measurement
Blood tests measure key hormones that reflect ovarian response to medications:
-
Estradiol (E2): The primary estrogen produced by developing follicles. Estradiol levels rise as follicles grow and reflect the magnitude of ovarian response. Normal rise is approximately 150-200 pg/mL per mature follicle. Excessively rapid increases (>100% daily) or very high levels (>2500 pg/mL) are concerning for OHSS risk.
-
Luteinizing Hormone (LH): Monitored to ensure no premature LH surge occurs before the trigger shot (which would prevent follicle maturation)
-
Progesterone: Checked to ensure no premature luteinization has occurred (progesterone should remain <1 ng/mL until after ovulation trigger)
-
Human Chorionic Gonadotropin (hCG): Measured after ovulation to confirm pregnancy
Together, these blood tests and ultrasound findings provide a comprehensive picture of ovarian response and enable real-time treatment optimization.
Interpreting Your Monitoring Results: Dose Adjustments and Treatment Decisions
Your fertility team’s interpretation of monitoring data directly guides treatment decisions:
If follicle growth is too slow:
-
Fewer follicles than expected, or follicles growing <2 mm per day
-
Action: Increase daily gonadotropin dose by 37.5-75 IU and continue daily or every-other-day monitoring
-
Rationale: The ovaries need more hormonal stimulation to respond appropriately
If the response is optimal:
-
1-4 follicles measuring 14-18 mm, mild-to-moderate estradiol elevation, normal endometrial thickness
-
Action: Continue current dose and monitor every 1-2 days
-
Rationale: The ovaries are responding as desired
If the response is excessive (OHSS Risk):
-
More than 4-6 follicles 12 mm or larger, estradiol levels >1500-2500 pg/mL, rapid daily estradiol increases >100%
-
Actions: May include dose reduction, coasting (temporarily stopping gonadotropins while monitoring continues), GnRH agonist trigger instead of hCG, or cycle cancellation if severe
-
Rationale: Prevent OHSS and reduce multiple pregnancy risk
The Trigger Decision:
The most critical decision is determining when to administer the trigger shot. The optimal criteria include:
-
At least 1-2 follicles measuring 17-20 mm in diameter (or per clinic-specific protocols)
-
Endometrial thickness ≥ 8 mm with preferably a trilaminar pattern
-
Estradiol levels in an appropriate range (typically 450-900 pg/mL, though higher ranges acceptable if multiple follicles)
-
Progesterone < 1 ng/mL (no premature luteinization)
-
Acceptable OHSS risk based on total follicle number and estradiol level
Timing of the trigger is critical because the LH surge naturally occurs 34-36 hours before ovulation. By administering hCG (which mimics the LH surge), the clinic ensures ovulation occurs in a predictable, controlled timeframe ideal for IUI or timed intercourse.
Triggering Ovulation and What Comes Next
The “Trigger Shot”: Human Chorionic Gonadotropin (hCG) Injection
Once ultrasound and bloodwork confirm that one or more follicles have reached optimal maturity, a “trigger shot” is administered. This injection contains Human Chorionic Gonadotropin (hCG), a hormone structurally and functionally similar to the natural LH surge that triggers ovulation.
Common hCG trigger preparations include:
-
Ovidrel (recombinant hCG, 250 mcg pre-filled pen)
-
Pregnyl or Novarel (urinary-derived hCG, requires reconstitution)
This shot initiates the final stage of egg maturation (completion of meiosis I and arrest in meiosis II) and reliably triggers ovulation to occur approximately 36-40 hours later, allowing for precise timing of conception efforts.
Alternative trigger options include:
-
GnRH agonist triggering (such as Lupron/leuprolide): Used in high-OHSS-risk patients; associated with significantly lower OHSS rates (1.9% vs. 11.8% with hCG) and may yield more mature oocytes
-
Dual trigger (hCG + GnRH agonist): Some evidence suggests improved oocyte maturation and reduced OHSS risk in selected patients
Your fertility team will recommend the most appropriate trigger based on your individual risk profile and cycle characteristics.
Post-Trigger Options: Maximizing Your Chances
After the trigger shot, the window for fertilization opens. Depending on your personalized treatment plan, couples will be advised on one of two primary options:
Option 1: Timed Intercourse
Couples are instructed to have intercourse on:
-
The day of the trigger shot
-
The day after the trigger shot
-
(Potentially the second day after trigger, depending on clinic recommendations)
This timing ensures sperm are present in the fallopian tubes when the egg is released, maximizing the chance of fertilization. Couples are typically advised to avoid intercourse between monitoring visits to ensure the partner can provide a fresh sample if switching to IUI.
Option 2: Intrauterine Insemination (IUI)
In an IUI procedure, a prepared sample of sperm is placed directly into the uterus using a thin catheter, passing through the cervix. This procedure:
-
Bypasses cervical and uterine factors that might impede sperm ascent
-
Places a high concentration of motile sperm very close to the released egg
-
Is timed to coincide precisely with ovulation, typically 34-42 hours after the hCG trigger (traditional timing 36 hours; some evidence suggests 42 hours may optimize outcomes)
-
Is a quick, office-based procedure lasting 2-5 minutes
-
Is generally well-tolerated with minimal discomfort
Following IUI, your doctor may recommend progesterone supplementation (via vaginal tablets, gels, or intramuscular injections) to support the corpus luteum and optimize endometrial receptivity for implantation.
Pregnancy Testing Timeline:
After IUI or timed intercourse, a home urine pregnancy test is typically performed approximately 10-14 days after ovulation, with blood beta-hCG testing to confirm positive results.
Managing Expectations and Treatment Outcomes
Success Rates with Ovulation Induction
Success rates for ovulation induction cycles vary based on multiple factors:
-
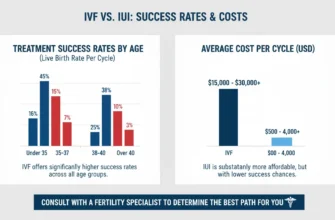
Live birth rates with IUI after ovulation induction: Approximately 12-19% per cycle with letrozole, 19-23% with clomiphene, and 19-32% with gonadotropins (depending on patient population and study)
-
Cumulative success rates: Most guidelines recommend limiting ovulation induction with IUI to 3-4 cycles before progressing to IVF, as success rates plateau and cumulative live birth rates after 4 cycles approach 40-50%
-
IVF success rates (for reference): 29-32% per cycle in women <40 years, with cumulative success approaching 65% after 6 cycles
Age is the most important prognostic factor, with significantly better outcomes in women under 35 years compared to those over 40.
When to Transition to More Advanced Treatment
Your fertility specialist should discuss progression to IVF if:
-
You have completed 3-4 ovulation induction/IUI cycles without pregnancy
-
You have a significantly diminished ovarian reserve (very low AMH, high FSH, low antral follicle count)
-
You have male factor infertility that may not respond well to IUI
-
You have tubal factor infertility (blocked tubes; IVF bypasses this issue)
-
You have endometriosis or other factors that may benefit from IVF
Conclusion
Ovulation induction with medications like letrozole and gonadotropins represents a cornerstone of modern fertility care, offering hope to countless individuals and couples facing infertility due to ovulatory disorders, particularly Polycystic Ovary Syndrome.
Letrozole has emerged as the highly effective and safe first-line oral treatment for PCOS-related anovulation, with superior live birth rates, lower multiple pregnancy risks, and an excellent safety profile compared to older medications. Injectable gonadotropins provide a more potent option for those requiring stronger ovarian stimulation, though they carry higher risks of OHSS and multiple pregnancies that require meticulous monitoring to mitigate.
The key to both successful and safe outcomes lies in:
-
Comprehensive fertility evaluation before treatment initiation
-
Individualized medication selection based on diagnosis and patient factors
-
Meticulous monitoring with ultrasound and bloodwork
-
Appropriate dose titration based on ovarian response
-
Realistic expectations regarding success rates and treatment timelines
If you are struggling with irregular cycles, anovulation, or have been diagnosed with an ovulatory disorder like PCOS, the first and most important step is to consult with a reproductive endocrinologist or fertility specialist. Together, you can explore which treatment path is most appropriate for your unique situation and begin your personalized journey toward conception.
Key Terminology Reference
Anovulation: Complete absence of ovulation within a menstrual cycle
Antral Follicle Count (AFC): Number of small follicles (2-9 mm) visible on ultrasound; reflects ovarian reserve
Anti-Mullerian Hormone (AMH): Hormone produced by ovarian follicles; used to assess ovarian reserve
Aromatase Inhibitor: Medication that blocks conversion of androgens to estrogen
Corpus Luteum: Remnant of the ovulated follicle; produces progesterone after ovulation
Endometrium: Inner lining of the uterus where embryos implant
Follicle-Stimulating Hormone (FSH): Pituitary hormone that stimulates ovarian follicle development
Gonadotropin: Hormone that stimulates gonad function (FSH and LH)
hCG (Human Chorionic Gonadotropin): Hormone produced by pregnancy; used to trigger ovulation
Intrauterine Insemination (IUI): Procedure placing sperm directly into the uterus
Luteinizing Hormone (LH): Pituitary hormone that triggers ovulation
Oligo-ovulation: Infrequent or irregular ovulation
OHSS (Ovarian Hyperstimulation Syndrome): Exaggerated response to ovulation induction medications
Ovulation Induction: Fertility treatment using medications to stimulate ovulation
PCOS (Polycystic Ovary Syndrome): Endocrine disorder characterized by irregular ovulation, elevated androgens, and insulin resistance
Transvaginal Ultrasound: Ultrasound examination performed vaginally for detailed pelvic imaging”